Protein Crystallization: Hanging & Sitting Drops Methods
This section provides an introduction to protein crystallization, including the sitting and hanging drops crystallization methods.
Preparing the Protein Sample for Crystallization
Protein crystallization begins with protein production, which includes cloning, expression, and purification. People often say that crystallization is an art. However, some general principles must still be followed, and most importantly, various methods have been developed to facilitate protein crystallization.
To ensure successful crystallization, a thorough characterization of the purified protein in solution is essential. The protein must be of high purity (better than 99%), stable, and monodisperse. Monodisperse means that the solution should not contain aggregates or denatured material and, ideally, should not have various oligomeric forms of the protein. Additionally, we want the protein to remain stable within the pH range and other buffer conditions we plan to use in future work. On our company website, you can find a more detailed description of the process of protein sample preparation for crystallization.
To understand the idea behind the techniques used in protein crystallization, we need to understand the process’s thermodynamics.
Thermodynamics of Crystal Formation
For a protein to crystallize, we must bring a solution to a supersaturated state. This process resembles the crystallization of regular table salt: we can slightly heat the salt solution and add as much salt as we can dissolve to create a saturated solution. Then, we let the glass containing the salt solution sit on the table until its temperature cools to room temperature. The solution is now supersaturated because the solubility of the salt at room temperature is lower than its solubility at the initially elevated temperature. While resting on the table, tiny salt crystals may form at the bottom of the cup. If the solution is very “clean,” meaning there are no small dust particles or similar impurities present in the cup (as impurities may promote nucleation and crystal growth), the solution can remain clear on the table for some time. However, since the supersaturated solution is thermodynamically unstable, even minor external factors, such as a slight vibration of the table, can trigger the crystallization process. This represents a phase transition in which the salt shifts from a soluble state to a crystalline state.
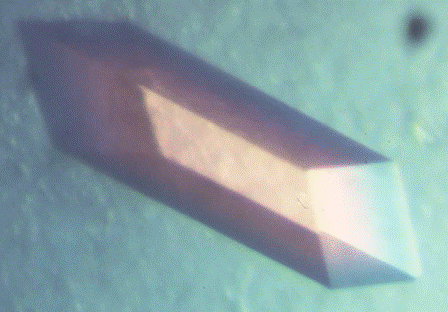

High-quality protein crystals were grown using both sitting and hanging drop setups.
Sitting and Hanging Drops Methods in Protein Crystallization
The simple technique for obtaining salt crystals, which involves dissolving salt in hot water, cannot be applied to protein crystallization. Proteins behave differently when heated and may easily lose their native structure (denature) and form aggregates. However, several other methods and tools have been developed to manage protein solutions and bring them into a supersaturated state. The easiest and most popular are the hanging and sitting drop crystallization methods, which are illustrated schematically in the image below:
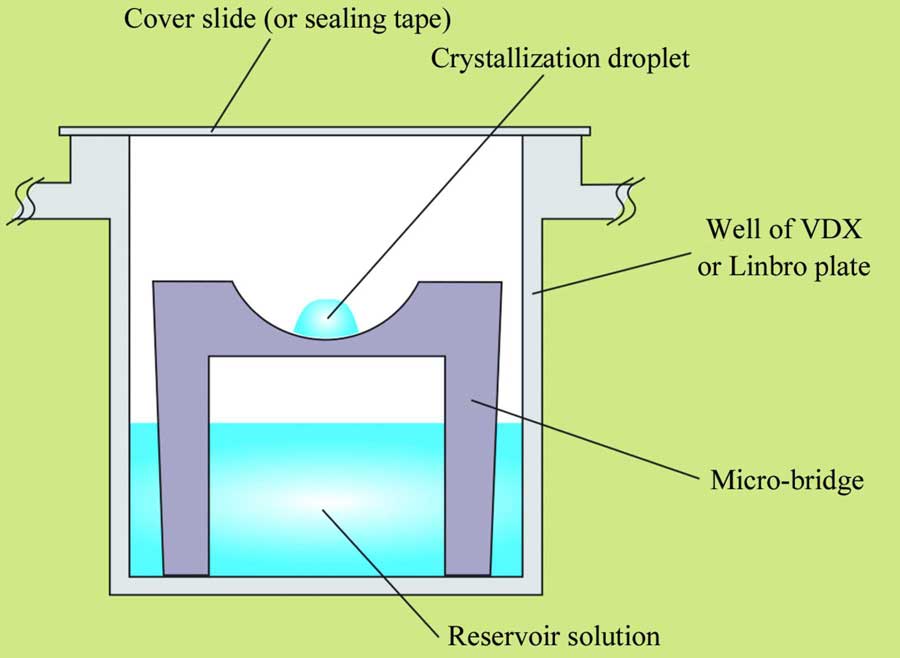
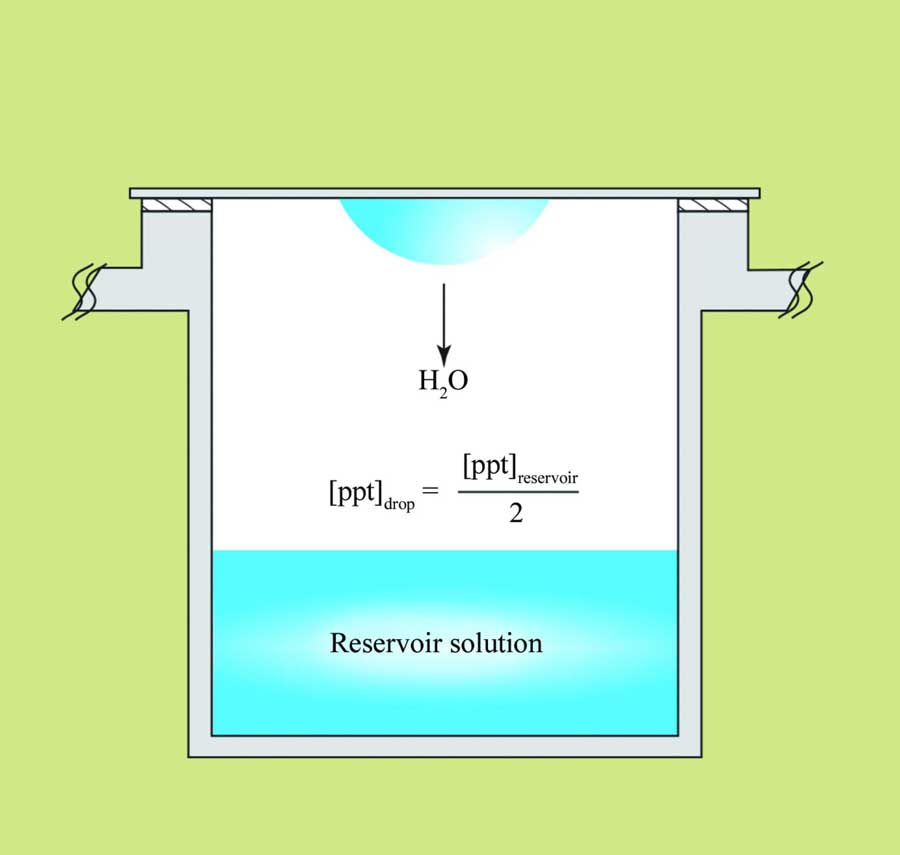
Sitting and hanging drop crystallization. Image from McPherson & Gavira, 2014. There are, of course, many other methods used for creating a concentration gradient for promoting supersaturation.
The sitting (left image) and hanging (right image) drop methods, also known as vapor diffusion methods, are the most widely used techniques in protein crystallization (see Table 2 in McPherson & Gavira for other crystallization methods). In this type of setup, the precipitant concentration in the reservoir solution (marked in the images) is higher than that in the drop. This creates a concentration gradient, leading to the evaporation of water from the drop into the reservoir, thereby reducing the drop’s volume and increasing the protein concentration. Numerous types of precipitants are employed in protein crystallization, the most common being polyethylene glycols (PEG) of various lengths, ammonium sulfate, and organic solvents. A detailed list of precipitants can be found in Table 3 of the review paper by McPherson & Gavira cited above. At some point, the protein concentration may reach a state of supersaturation. If all other parameters, such as pH, ionic strength, type of buffer, additives, and so forth, are suitable, the protein may begin to form crystals (crystallize). Some factors that affect protein solubility are listed at the bottom of this page.
Screening for Crystallization Conditions
Suppose we have a new protein to crystallize and are unsure of the exact conditions for crystallization, such as the type of buffer, pH, salt concentration, type of precipitant, or the optimal crystallization temperature. In that case, we need to screen for crystallization conditions. Initially, we need to test various combinations with different buffers, salts, the potential effect of various ligands (small molecules that may bind to the protein), and different precipitants. The precipitant is the main component responsible for the creation of the “concentration gradient” required in vapor diffusion and other types of crystallization methods. Below is a table listing some other factors that affect protein solubility. I have also downloaded an example of a screen offered by Hampton Research to show the large number of salts and precipitants used in this screen alone. And there are hundreds of other screens! This should give an idea of how protein crystallography works – many ready-made screens are tested before the right crystallization conditions are found!
Specialized liquid handling robotics and dedicated crystallization plates (image to the left below from Hampton Research) are used to set up nano-liter sitting-drop crystallization screens (image below showing mosquito protein crystallization robot). An example is the Mosquito liquid handling robot that is used with 96-well plates (images below). A liquid handling robot may need as little as 15 μL of protein sample to screen 96 different crystallization conditions. The process is speedy and takes a couple of minutes. However, the initial conditions obtained from these screens often require further optimization before crystals suitable for X-ray data collection are produced.
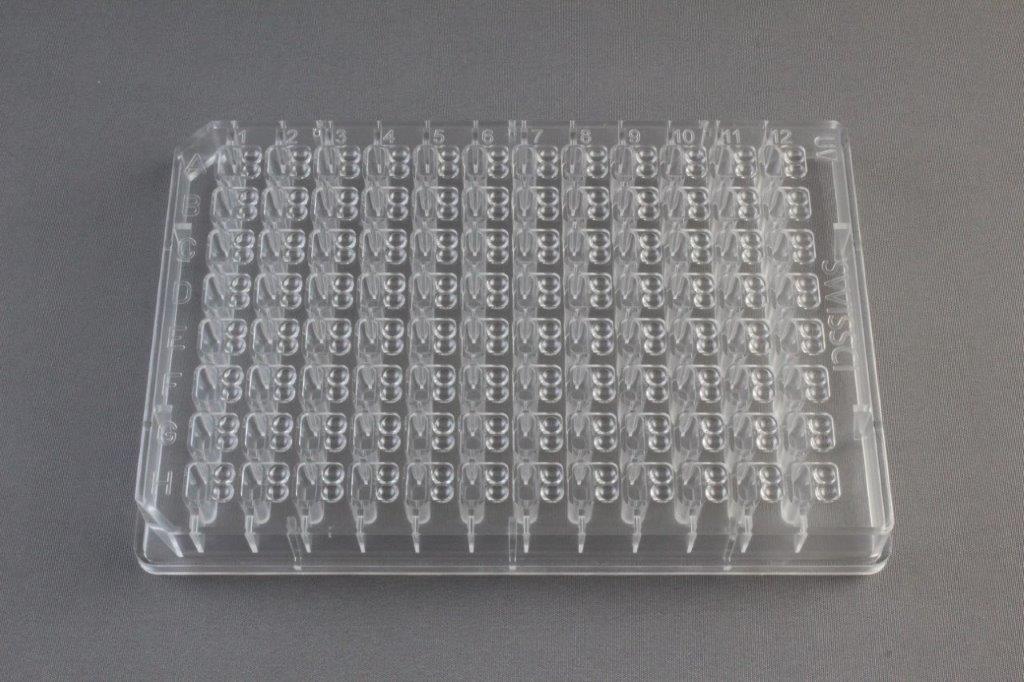

The protein concentration to be used in screening is also an essential factor to consider and depends on the solubility of the protein. As a rule of thumb, the higher the solubility of the proteins, the higher the concentration should be. Remember that we need to reach a supersaturation level! The range between 5 and 10 mg/ml is common for most proteins. It is also possible to assess the required initial concentration using a so-called pre-crystallization test (PCT), for example, from Hampton Research.
Common Factors that Affect Protein Solubility
1) pH
2) Ionic strength
3) Concentration of precipitant
4) Concentration of macromolecule
5) Temperature
6) Additives, effectors, and ligands
8) Organism source of macromolecules
9) Presence of substrates, coenzymes, inhibitors
10) Reducing or oxidizing environment
11) Metal ions
12) Rate of equilibration
Even gravity has been considered as one of the possible protein crystallization parameters! The list may be extended, and many more factors affecting protein solubility may be added.