Structure-Based Drug Design of COX-1 and COX-2 Specific Inhibition
The design of specific inhibitors for COX-1 and COX-2 has become a classic example of structure-based drug design. In this analysis, we examine the sequence, structure, and ligand binding to the COX enzymes. We demonstrate how small differences in the structure of the active sites of the two enzymes were utilized to design specific inhibitors.
Introduction
As discussed earlier in the introduction to structure-based drug design, the most straightforward strategy for drug discovery is to identify a biological process or biochemical pathway we aim to interfere with, determine the molecular components (i.e., proteins, DNA, RNA, etc.) involved in that pathway, and select the target for drug design from among those components. Subsequently, depending on the available information, such as the known three-dimensional structure of the protein and the ligand complex, devise a strategy for the overall project. The example here illustrates a scenario where the available structural information was crucial in guiding the design of specific inhibitors targeting two closely related proteins, prostaglandin cyclooxygenases 1 and 2 (COX-1 and COX-2).
Prostaglandin Biochemistry: COX-1 & COX-2
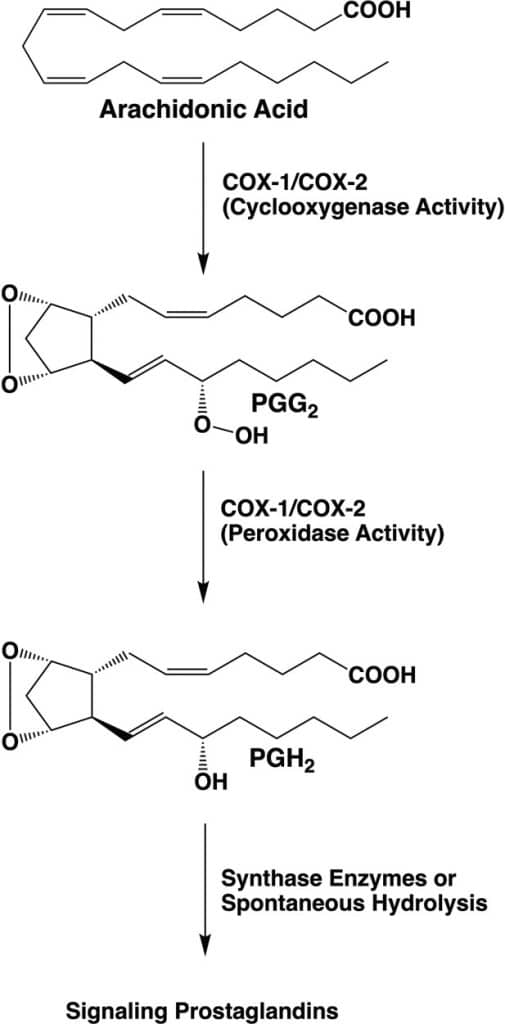
Prostaglandins were discovered in the 1930s by the Swedish biochemist Ulf von Euler. They were named “prostaglandins” because von Euler found them in human semen and believed they were produced in the prostate gland. Further research revealed that they are produced in almost all tissues of both males and females. Prostaglandins belong to the class of lipid signaling molecules and regulate many cellular processes through the activation of G-protein coupled receptors (GPCRs, chemistry Nobel Prize in 2012 to Robert Lefkowitz and Brian Kobilka). The precursor for prostaglandin synthesis in cells is arachidonate, which is released from membrane phospholipids by the action of phospholipase A2, activated by a GPCR receptor. Arachidonate is the substrate of prostaglandin cyclooxygenases 1 and 2 (COX-1 and COX-2). The COX enzymes play a key role in lipid signaling by catalyzing the first committed step in prostaglandin synthesis. They host two activities, the first is oxygenase, which uses 2 O2 to oxygenate arachidonate, while the second is peroxidase, which removes one of the oxygens (image below).
COX-1 and COX-2 share approximately 60% sequence identity and a conserved three-dimensional structure. They are also related biochemically and contain cyclooxygenase and peroxidase sites. However, they have different functions in organisms. COX-1 is expressed in most tissues and, among other things, produces the prostaglandins required to suppress acid secretion in the stomach and promote the formation of a protective mucus coating of the stomach wall. COX-2 expression, on the other hand, is induced by growth factors, inflammatory agents, and tumor promoters. When expressed, COX-2 produces prostaglandins associated with pain and fever.
Well-known non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, paracetamol, and ibuprofen inhibit COX-2 and thereby inhibit inflammation-related prostaglandin production. However, these compounds also inhibit COX-1, which may result in side effects such as gastrointestinal bleeding and ulcers. When this became known, a search began for specific compounds that could selectively inhibit COX-2 without inhibiting COX-1. The X-ray structures of complexes of COX-1 and COX-2 with inhibitors provided crucial insights that contributed to a better understanding of the factors that control ligand specificity.
The cyclooxygenase enzyme reaction
The two-step reaction catalyzed by COX1 and COX2 involves dioxygenation and cyclization of arachidonic acid at the cyclooxygenase active site of COX-1 or COX-2, which yields PGG2. Subsequent reduction of the 15-hydroperoxyl group of PGG2 at the peroxidase active site yields PGH2.
PGH2 serves as a substrate for five different synthases, producing four signaling prostaglandin products (PGE2, PGI2, PGF2α, and PGD2) or thromboxane A2 (TXA2). Rouzer CA & Marnett LJ (2020). Chem. Rev. 120, 15, 7592-7641.
The Structure of COX-1 & COX-2. Substrate Binding
Before starting a drug discovery project, we first need to analyze the target’s three-dimensional structure and examine its ligand binding mode. Due to the essential physiological role played by the COX enzyme and the pharmaceutical industry’s interest, the structures of many COX-1 and COX-2 X-ray crystallographic complexes with various ligands (substrates and inhibitors) have been determined. I recommend the excellent review paper by Rouzer and Marnett, mentioned above, which discusses the available structures and inhibitors. Here, we are primarily interested in the factors that made the design of COX-2-specific inhibitors possible.
In this type of project, we always need to start with a closer look at the sequences of the two proteins. The image below shows an alignment of COX-1 and COX-2 from three different organisms.
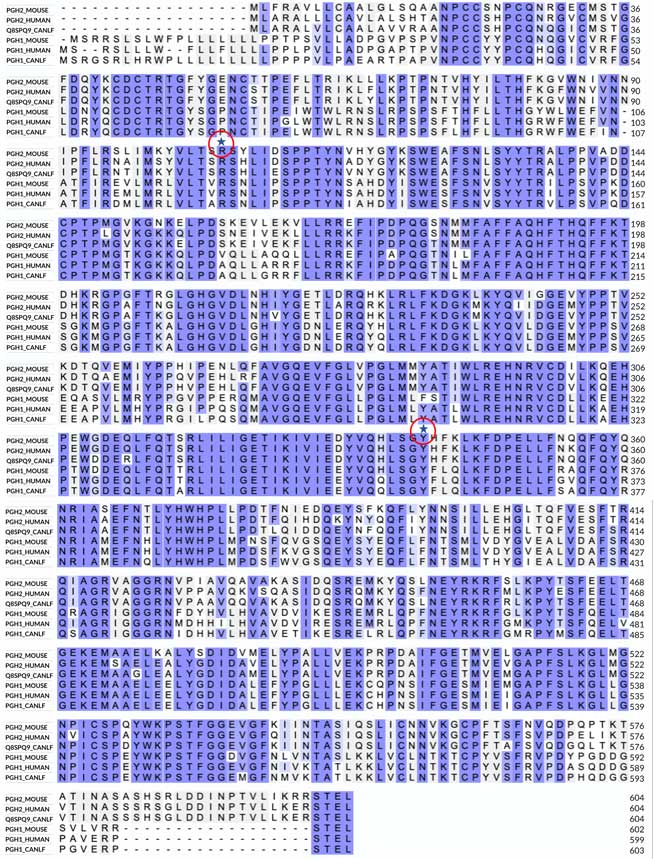
The alignment reveals no significant insertions or deletions in the three sequences. The invariant regions in the alignment are boxed, suggesting a highly conserved three-dimensional structure. The most notable differences between COX-1 and COX-2 include COX-1’s longer N-terminal sequence and COX-2’s longer C-terminal sequence, which maintain a similar total length of the polypeptides. I have also highlighted two invariant residues in the alignment: a Tyr and an Arg. As discussed below, these residues are critical for binding a substrate and many NSAID inhibitors that contain a carboxyl group.
The Three-Dimensional Structure of COX Enzymes
The left image below shows a ribbon representation of the COX-1 dimer in a complex with arachidonic acid (PDB ID 1DIY). The CATH database recognizes two domains in each monomer: the small β-hairpin domain (shown in yellow), which belongs to the laminin superfamily (CATH Superfamily 2.10.25.10). This fold is found in many proteins with diverse functions and exhibits low structural conservation. The large helical domain (red color) belongs to the heme peroxidase family (CATH Superfamily 1.10.640.10, orthogonal bundle) and demonstrates a high degree of structural conservation. The smaller domain is positioned between the two enzyme subunits, presumably stabilizing their interactions. The large domain contains the two catalytic sites and binds the heme prosthetic group (shown in the space-filling model in the image), which is located between the cyclooxygenase and peroxidase sites. The heme is required for both enzyme reactions, although to form a complex with the substrate, the molecule in this structure is Co-protoporphyrin IX, chosen because it does not support the enzymatic reaction. The helices at the bottom of the structure (residues 73-116) run parallel to the lipid membrane and anchor the dimer to the lipid bilayer. The sequence alignment shows that this region is among the least conserved. As usual, clicking on the image will take you to the PDB page, where you can closely examine the structure in the graphics.
Substrate Binding to COX-1
The right image shows a close-up of arachidonic acid bound in the active site of COX-1. The position of the bound arachidonic acid is also shown on the left image as a green space-filling model inside the structure. I have marked some of the active site residues mentioned in the text. As expected, the binding site is prolonged and highly hydrophobic (the substrate makes 43 hydrophobic contacts with the protein). The invariant Tyr355 and Arg120 at the entrance to the tunnel (also marked on the sequence alignment) interact with the carboxylic group of the substrate or with the polar groups of other ligands, including NSAID inhibitors. The spacious binding site explains the structural diversity of COX substrates and inhibitors. Interesting to note that the binding site of COX-2 is about 25% bigger, which, as will be shown below, has implications for the design of COX-2-specific inhibitors.
In the middle of the tunnel lies Ser530. This residue is the target for acetylation by aspirin, one of the most well-known inhibitors of COX enzymes. Acetylation blocks the enzymatic reaction catalyzed by COX-1 and COX-2, and as you might expect, aspirin reacts equally well with both enzymes. Although Ser530 does not participate in the enzymatic reaction, replacing it with a residue that has a larger side chain inactivates the enzyme, suggesting that the correct positioning of the substrate at this location is essential for the reaction (reviewed in Rouzer & Marnett, 2020) mentioned above. Opposite to Ser530 is Tyr385, a critical residue for the cyclooxygenase reaction. It donates a hydrogen atom to the heme during enzyme activation, which generates a tyrosyl radical that attacks the substrate’s hydrogen atom.
Inhibitor Binding to COX-1
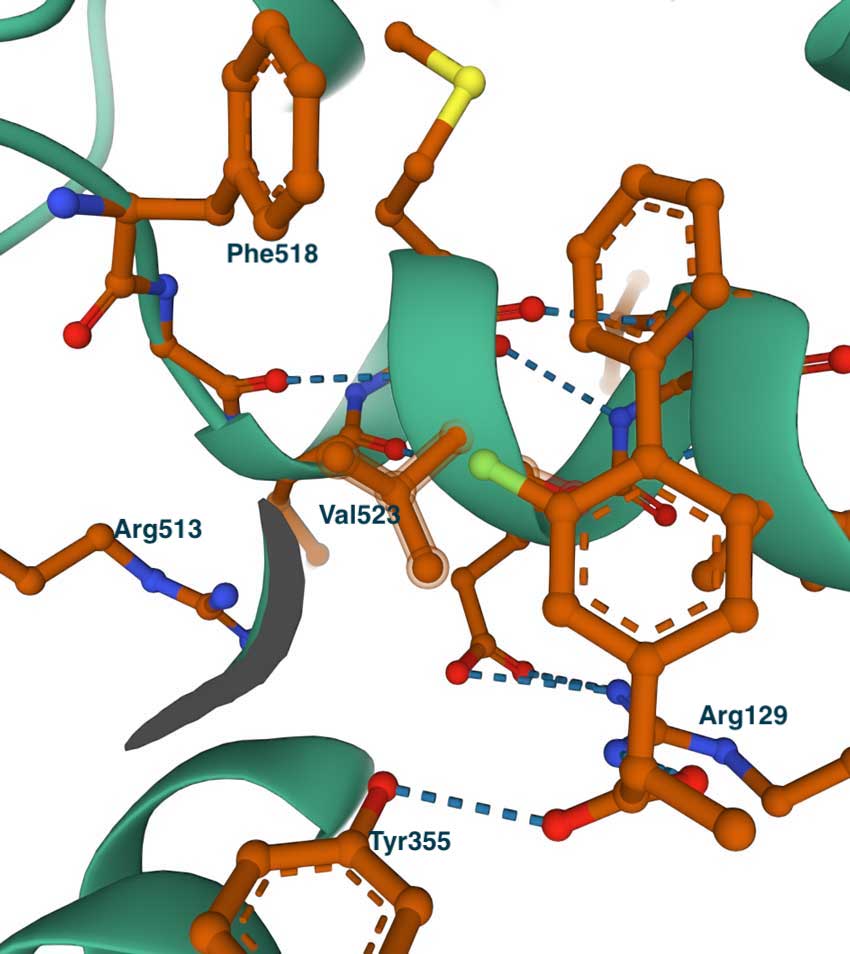
The large size of the substrate-binding pocket of the COX enzymes enables the design of a wide variety of small-molecule inhibitors. Structural studies show that most inhibitors occupy the entrance and central part of the substrate tunnel, often referred to as the proximal and central binding pockets of COX-1 and COX-2. Similar to the substrate, many ligands interact with Tyr355 and Arg120 at the entrance of the tunnel. In addition to the proximal and central pockets, COX-2-selective inhibitors have been found to occupy the so-called side pocket. This side pocket is created by the substitutions of Ile434, Ile523, and His513 of COX-1 to Val434, Val523, and Arg513 in COX-2, all located near the active site. The Ile523Val substitution is likely the most important because it provides access to the substrate binding tunnel.
To illustrate the effect of these observations, we will examine the X-ray structures of the complexes formed by the known anti-inflammatory drug flurbiprofen with COX-1 and COX-2. Next, we will compare the flurbiprofen complex to the complex of the COX-2-selective inhibitor SC-558. The complexes of flurbiprofen and SC-558 were published in a Nature paper from 1996 (Kurumbail et al., 1996). Flurbiprofen is a weakly selective inhibitor with IC50 values of 0.29 μM for COX-1 and 2.56 μM for COX-2.
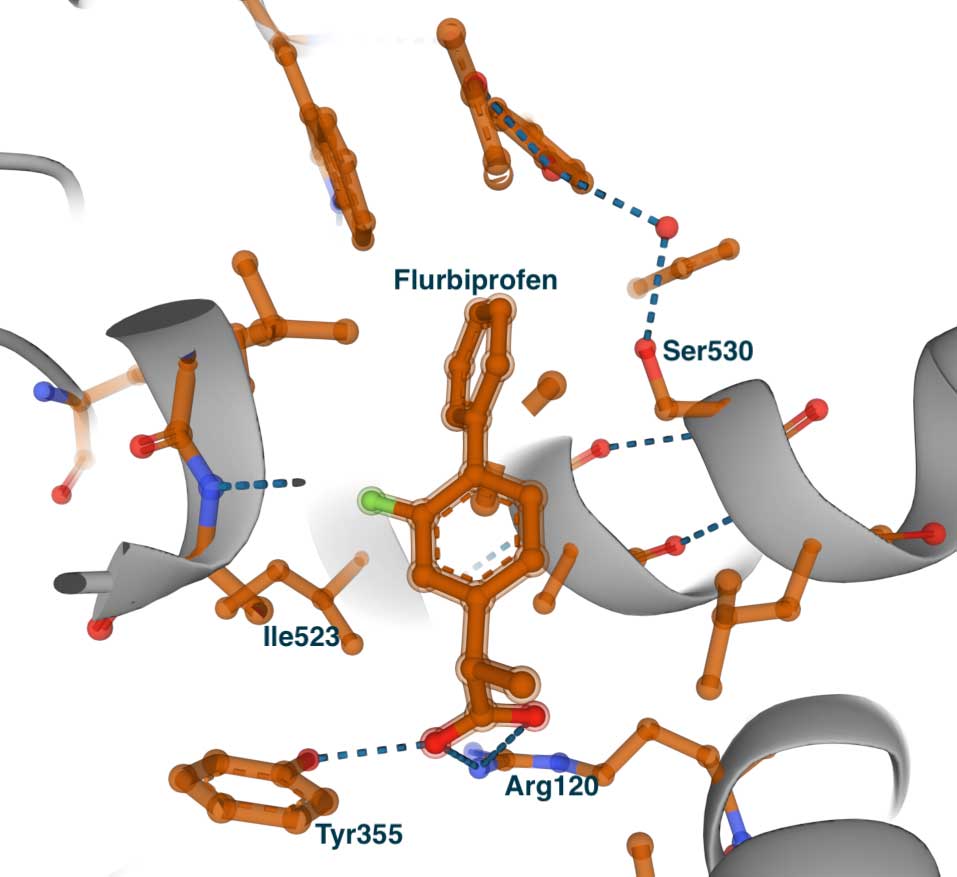
The left image below shows the complex of flurbiprofen with COX-1 (PDB id 1CQE). The inhibitor is extended from the entrance, where it makes contact with Tyr355 and Arg120, to the central pocket and Ser530. Some residues that line the side pocket are shown – Phe518, His513, His90, Tyr355, and Ile523. To illustrate the concept of the side pocket, the figure on the right presents a slightly rotated view of the left image. On the right, we can see that the methyl group of Ile523 blocks the entrance to the pocket where His513 can be seen. This becomes especially clear when we examine the complex of flurbiprofen with COX-2.
Inhibitor Binding to COX-2
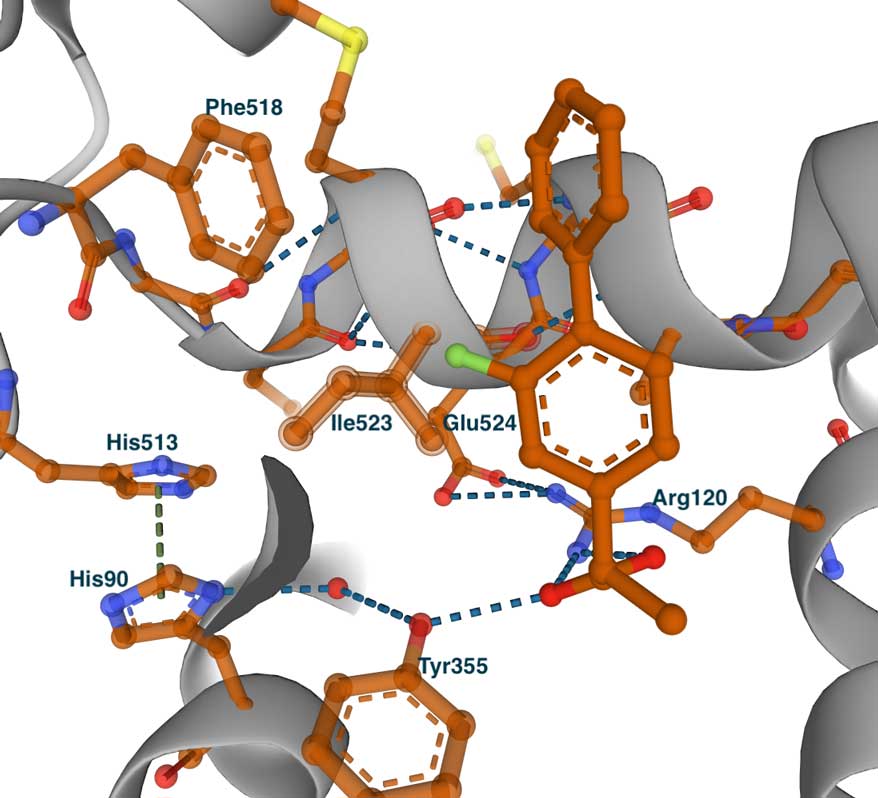
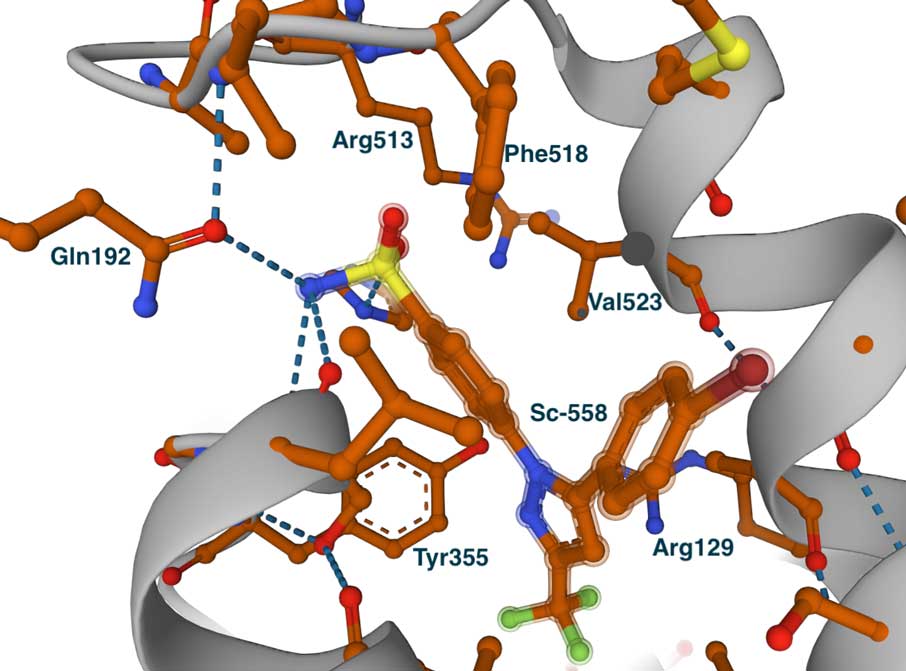
This image on the left shows the COX-2 complex with flurbiprofen (PDB id 3PGH), highlighting Val523 and Arg513 (Ile523 and His513 in COX-1). Compared to the COX-1 complex above, there is a wider opening towards the side pocket. Although not depicted in this image, the Ile434Val substitution causes a shift in the position of Phe518 and nearby residues, broadening the pocket and accommodating larger groups. This is evident in the image on the right, which displays the bound COX-2-specific diarylheterocycle class inhibitor SC-558 in complex with COX-2 (PDB ID 6COX). You can also view the complete list of interactions made by this molecule in the LigPlot chart at PDBsum. In this complex, the phenyl sulfonamide group (green atom) occupies the side pocket of COX-2, at hydrogen bonding distance from Gln192, His90, Ser353, and the main chain carbonyl of Leu252. The other groups of the inhibitor—the pyrazole and bromophenyl rings—occupy the proximal and central binding pockets, respectively. This binding pose is similar to that of other inhibitors, such as flurbiprofen.
Concluding remarks
This brief story illustrates how minor structural differences can be leveraged to achieve specificity in inhibitor design, particularly regarding COX-1 and COX-2 inhibitors. The key takeaway is that these methods are most effective when we understand the details of the protein we are targeting, along with its three-dimensional structure. Often, we may need meticulous studies of enzyme kinetics and comprehensive ligand binding studies, supplemented with mutations, to uncover the roles of various active site residues. Additionally, we may need many structures of ligand complexes with the protein. In a later chapter, you can find a more detailed presentation of the basics of X-ray crystallography. A more detailed account of the COX-1 and COX-2 story can be found in the review cited earlier by Rouzer and Marnett (2020).

